By Vanmeeganathan Balakrishnan | May 13, 2024 | Ontario, Canada
Overview:
Artificial intelligence (AI) has come to be a disruptive force in many different sectors, transforming processes and opening up new opportunities. AI is about to completely change the Computer System Validation (CSV) and Computer System Assurance (CSA) landscape in the pharmaceutical industry.
Pharmaceutical companies can now more than ever streamline validation procedures, improve data integrity, and guarantee regulatory compliance with the help of AI algorithms. In this blog, we delve into the role of AI in CSV and CSA, exploring its potential to revolutionize these critical aspects of pharmaceutical operations and pave the way for enhanced compliance and efficiency.
Defining Computer System Validation (CSV) and Assurance (CSA) in Pharma & Life Sciences
Computer System Validation (CSV) and Computer System Assurance (CSA) are essential processes in the pharmaceutical industry to ensure the reliability, integrity, and compliance of computerized systems used in various aspects of drug development, manufacturing, and distribution. Here’s a brief definition of each:
Computer System Validation (CSV):
CSV is a systematic and documented process used to ensure that computerized systems, including hardware, software, and associated processes, meet predefined requirements and are suitable for their intended use in regulated environments.
CSV involves rigorous testing, documentation, and validation activities throughout the lifecycle of a computer system, from initial development and implementation to ongoing maintenance and retirement.
The primary goal of CSV is to demonstrate that a computer system operates consistently and reliably within specified parameters, while also ensuring data integrity, security, and compliance with regulatory standards.
Computer System Assurance (CSA):
CSA encompasses a broader set of activities aimed at providing confidence and assurance in the reliability, security, and performance of computer systems throughout their lifecycle. While CSV focuses primarily on the validation of individual systems, CSA extends beyond validation to encompass ongoing monitoring, maintenance, and continuous improvement of systems to ensure their effectiveness and compliance.
CSA includes proactive measures to identify, assess, and mitigate risks associated with computerized systems, as well as the implementation of controls, safeguards, and best practices to enhance their reliability, resilience, and security.
The ultimate objective of CSA is to ensure that computer systems operate effectively, consistently, and securely to support the goals and objectives of the organization while maintaining compliance with regulatory requirements.
The Role of AI in CSV and CSA:
In the pharmaceutical sector, artificial intelligence (AI) technologies are completely changing Computer System Validation (CSV) in pharma and Computer System Assurance (CSA) processes. AI algorithms can help companies improve data integrity, automate validation processes, and guarantee regulatory compliance. From automated test case generation to real-time monitoring and anomaly detection, AI plays a crucial role in optimizing CSV and CSA processes for improved efficiency and effectiveness.
Benefits of leveraging AI for enhanced compliance in regulated environments:
AI has numerous benefits for improving compliance in regulated settings. Through the automation of repetitive activities, real-time analysis of large datasets, and anomaly detection made possible by AI technology, CSV and CSA processes become more accurate, efficient, and reliable. Organizations can enhance patient safety and product quality in the end by using AI to automate validation processes, save human labor, and guarantee regulatory compliance.
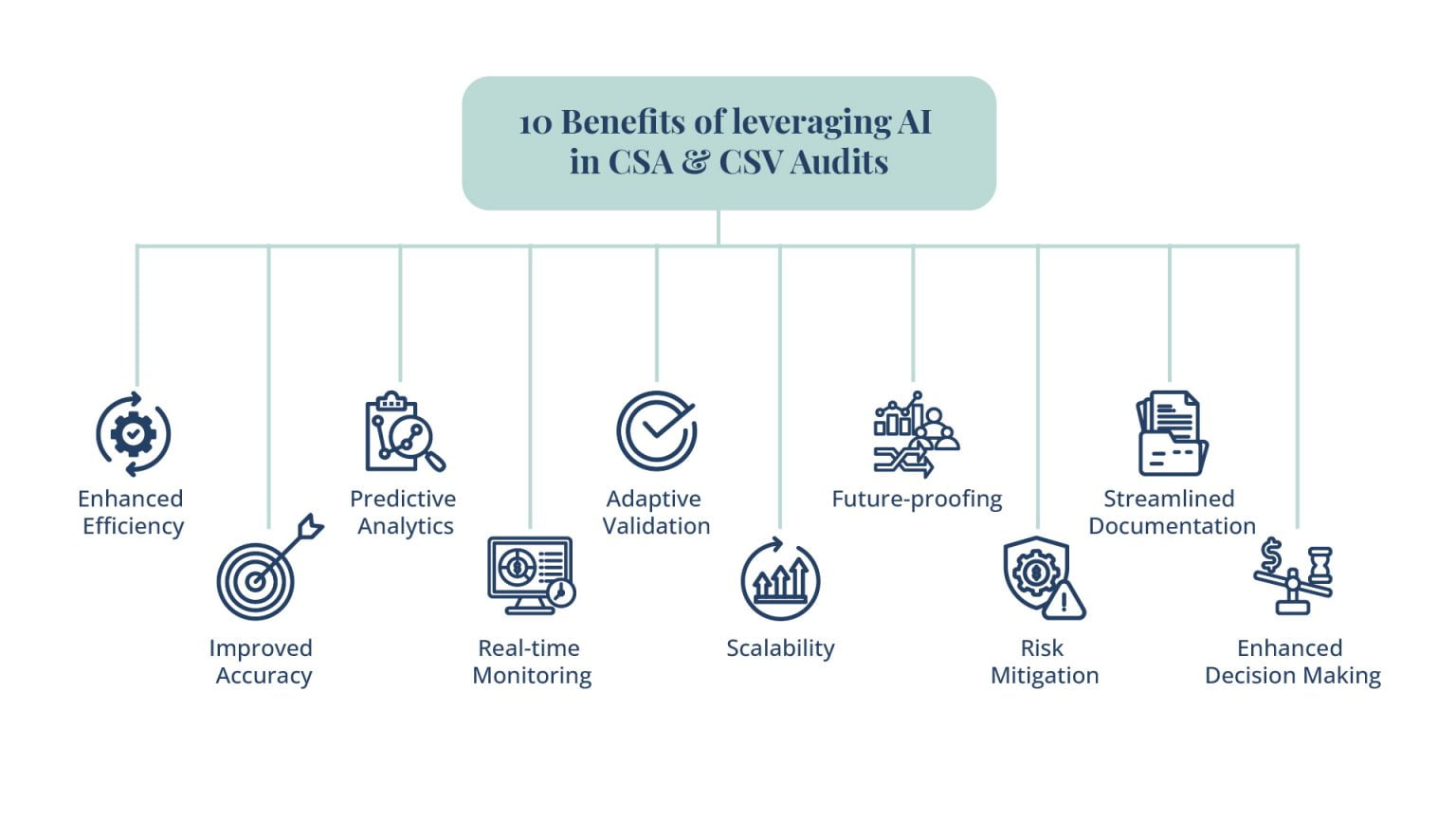
Understanding how AI technologies can revolutionize CSV and CSA processes:
AI technologies can automate laborious activities, analyze huge datasets, and offer insightful information about system performance and compliance. Hence, they have the potential to completely transform Computer System Validation (CSV) and Computer System Assurance (CSA) processes. With AI algorithms, organizations can enhance the efficiency, accuracy, and reliability of CSV and CSA processes, resulting in better outcomes and ensuring compliance with regulatory requirements.
Here are some of the fields where AI can revolutionize CSA and CSV practices in the Pharma and Life Science industry.
1. AI-powered Data Analysis and Predictive Modeling:
AI algorithms can analyze large datasets to identify patterns and trends, enabling organizations to make data-driven decisions and predict system behavior. With predictive modeling techniques, organizations can forecast potential compliance issues and proactively address them before they escalate.
AI-powered data analysis and predictive modeling play a crucial role in enhancing the efficiency and effectiveness of CSV and CSA processes, ultimately ensuring compliance with regulatory requirements.
2. Automated Test Case Generation and Execution:
AI-driven tools can automatically generate test cases based on system requirements, enabling organizations to accelerate validation processes and ensure thorough testing coverage. By automating test case execution, organizations can reduce manual effort, minimize human error, and improve efficiency in CSV and CSA activities.
Automated test case generation and execution streamline validation processes, enabling organizations to meet regulatory requirements and achieve compliance more effectively.
3. Real-time Monitoring and Anomaly Detection:
Artificial Intelligence-enabled monitoring systems can track system performance and data integrity in real time, spotting anomalies and deviations. Organizations can reduce the possibility of non-compliance and guarantee patient safety and product quality via early detection of compliance problems.
Effective CSV and CSA procedures require real-time monitoring and anomaly identification in order for companies to stay in compliance with legal requirements.
4. Streamlining Documentation and Reporting:
AI-powered document management, version control, and report generating solutions optimize documentation and reporting procedures. They help in increasing accuracy and efficiency in CSV and CSA operations. By automating tedious tasks such as document organization, version tracking, and report generation, organizations can reduce manual effort and ensure compliance with regulatory requirements.
Automation of reporting and documentation using AI-powered solutions allows businesses to concentrate on their main business operations while also adhering to legal requirements.
5. Regulatory Considerations and Compliance Standards:
Companies working in regulated environments must be aware of the regulatory rules and standards that apply to CSV and CSA, such those established by the FDA, EMA and GAMP.
Artificial intelligence (AI) solutions can automate validation procedures, guarantee data integrity, and offer real-time insights into system performance and compliance, therefore helping businesses comply with regulations. Organizations can reduce risks, keep compliance, guarantee patient safety and product quality, and so align with regulatory issues and compliance standards.
To sum up, by embracing AI-driven approaches, organizations can streamline validation processes, enhance data integrity, and ensure compliance with regulatory standards more effectively than ever before.
As you embark on your journey towards leveraging AI in CSV and CSA, remember that RxCloud is here to support you every step of the way. Contact us today to discover how our innovative solutions can help you unlock the full potential of your practices and achieve unparalleled success in pharmaceutical operations.
