By Vanmeeganathan Balakrishnan | May 13, 2024 | Ontario, Canada
Introduction
In the stringent world of pharmaceutical manufacturing, adherence to Good Manufacturing Practices (GMP) is not just regulatory, it’s crucial for ensuring the quality and safety of products. Regular GMP audits are essential for compliance and maintaining the highest standards of production. These audits help in identifying areas that require improvements and ensuring that the products manufactured meet the safety requirements necessary for public health.
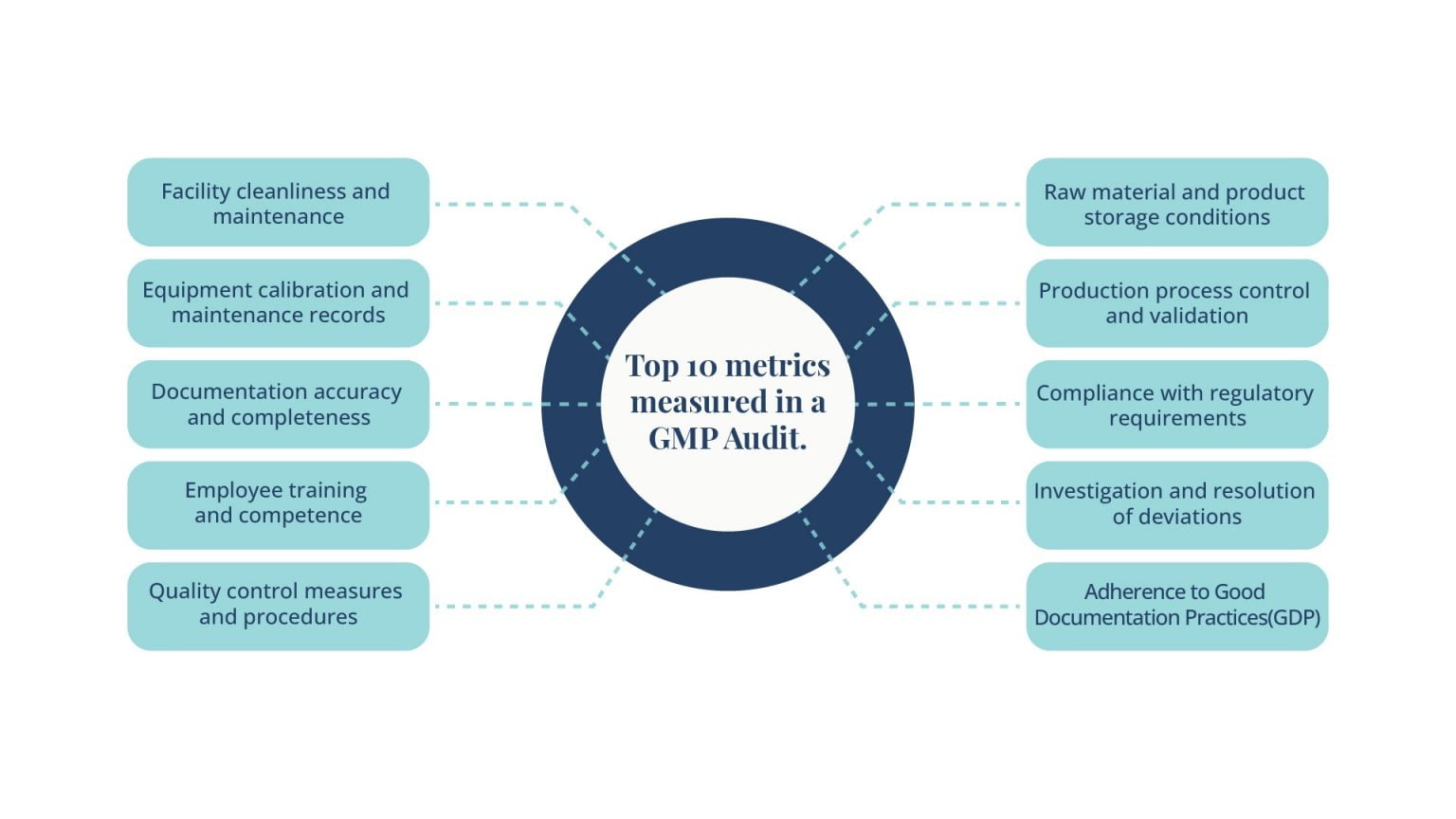
Here, we break down the process into eight simple, actionable steps that can make your GMP audit both thorough and effective.
Step 1: Define the Audit Scope and Objectives
The first step in a successful audit is to clearly define its scope and objectives. What specific processes or areas should the audit cover? What are the goals, risks and criticalities of the vendor? Setting a clear direction and purpose helps in focusing the audit efforts and ensuring comprehensive coverage of necessary aspects.
Step 2: Prepare an Audit Checklist
Creating a detailed checklist based on GMP standards and specific regulatory requirements is essential. This checklist will guide the audit process, ensuring that all critical elements, such as SOPs, compliance with safety regulations, and staff qualifications, are systematically reviewed.
Step 3: Select a Qualified Auditor
The effectiveness of an audit greatly depends on the expertise of the auditor. Select someone with extensive knowledge and experience in GMP practices and the specific type of manufacturing facility being audited. Their understanding of regulatory nuances can make a significant difference in the depth of the audit.
Step 4: Conduct Pre-Audit Activities
Before the on-site audit, engage in thorough pre-audit activities. This involves reviewing relevant documentation, quality control records, and conducting preliminary interviews with key personnel. Keep a handy checklist with relevant regulations and tailor the list based on the product, risk, and criticality of the audit/facility. These activities help in identifying potential compliance gaps and streamlining the focus areas for the on-site audit.
Step 5: Execute the On-Site Audit
During the on-site visit, utilize your checklist to systematically review each area. Inspect facilities and equipment for cleanliness and upkeep, assess material handling for compliance, and verify that production procedures and quality controls are rigorously followed and documented. Ensure thorough examination of packaging, labeling, and personnel training, alongside quality assurance practices including complaint and recall management. Observe operations closely, interview staff, and document findings meticulously to ensure a comprehensive evaluation of compliance with good manufacturing standards.
Step 6: Identify and Document Findings including CAPA Recommendations
During this audit phase, identify and document all non-compliance issues, categorizing them by severity to prioritize corrective and preventive actions (CAPA). Recommend specific CAPA measures to address both immediate issues and prevent future occurrences, ensuring these actions are clear and actionable. Documentation should be clear and structured with detailed key findings, thus facilitating effective implementation and continuous improvement in quality assurance practices.
Step 7: Report Audit Findings and Recommendations
Compile a detailed audit report summarizing all findings and explicitly outlining CAPA. Include a plan for tracking CAPA implementation and effectiveness, specifying key performance indicators and timelines. The report should detail the process for follow-up reviews to verify CAPA effectiveness and outline the criteria for audit closure, which includes successful CAPA resolution. This structured approach ensures comprehensive resolution of issues and long-term compliance improvements.
Step 8: Implement Corrective Actions and Verify Effectiveness
Finally, collaborate with the audited entity to address the findings. Establish corrective measures and a timeline for implementation. Follow-up monitoring or subsequent audits should be planned to ensure these actions are effective and that compliance is maintained over time.
Conclusion
A well-planned and executed GMP audit is a powerful tool for ensuring pharmaceutical products are manufactured under conditions that lead to high-quality outputs. By adhering to these eight steps, companies can not only comply with regulatory requirements but also demonstrate their commitment to quality and safety. Remember, the ultimate objective of GMP audits is to safeguard the consumer by ensuring that all products are produced consistently and are exactly what they purport to be.
Embarking on your GMP audit with these steps in mind will ensure a thorough and effective process, contributing significantly to your quality assurance goals and ultimately, to public health and safety.
FAQ’s
1. What is a GMP audit?
In the life sciences industry, a GMP (Good Manufacturing Practice) audit is a formal examination conducted to verify that a manufacturing facility complies with GMP regulations, ensuring that products (pharmaceutical drug, biotechnology products, medical devices etc.) are consistently produced and controlled according to regulatory and quality standards.
2. Why are GMP audits important?
GMP audits are crucial because they ensure that manufacturers are adhering to the quality standards necessary to produce safe and effective pharmaceutical products. They help identify areas of improvement, reduce the risk of product recalls or compliance issues, and protect public health.
3. Who should perform a GMP audit?
A GMP audit should be performed by a qualified auditor with extensive knowledge and experience in quality management systems, the specific manufacturing processes, and the regulatory requirements applicable to the products being manufactured.
4. How often should GMP audits be conducted?
The frequency of GMP audits can vary based on several factors, including the complexity of processes, previous audit findings, changes in production or quality processes, regulatory requirements, and industry best practices. Typically, it is recommended to conduct GMP audits annually, but more frequent audits may be necessary depending on the circumstances.
5. What should be included in a GMP audit report?
A GMP audit report should include a comprehensive summary of the audit findings, including observations of non-compliance, recommendations for corrective and preventive actions (CAPA), and a plan for CAPA implementation and follow-up. It should also detail the audit scope, criteria, methodology, and any conclusions drawn from the findings.
6. What happens if non-compliance issues are found during a GMP audit?
If non-compliance issues are identified, the auditor will recommend corrective actions to address the specific issues and preventive actions to avoid recurrence. The manufacturer is required to implement these actions promptly and may be subject to follow-up audits to ensure compliance and the effectiveness of the implemented measures.
7. Can a GMP audit be failed?
Yes, a GMP audit can be failed if critical non-compliance issues are found and the manufacturing facility does not meet the required regulatory standards. Failure may result in a variety of consequences, including regulatory actions, product recalls, or cessation of production until issues are resolved.
Contact us today to understand how we can help make your next GMP audit a seamless success!
